Abstract
Background Activated protein C (APC) is a pleiotropic coagulation protease with anticoagulant, anti-inflammatory, and cytoprotective activities. Antibodies that can selectively modulate these APC activities could contribute to the understanding of the regulation of these physiological mechanisms and the development of therapeutics for the pathologies associated with these pathways
Aim We sought to construct an antibody library consisting of small-sized nanobodies that could target neo-epitopes on the exosites of the activated form of protein C that are potentially inaccessible or whose three-dimensional structure is not recognized by conventional antibody formats. These APC-specific nanobodies will be used to determine the effect of the blockade of different exosites on APC pleiotropic activities.
Methods A nanobody phage library specific for APC exosites was derived from peripheral blood mononuclear cells of llamas immunized with active site-blocked APC (APC-PPACK). The nanobody library was panned for APC-specificity. Biolayer interferometry (BLI) was used to determine the KDs and to epitope bin nanobodies into different clusters. APC functional assays, including Protac-APTT clotting assays, histone H3 cleavage assay, and SEAP-PAR1 cleavage assay with HEK293 cells, were employed to characterize the effects of the nanobodies on APC's pleiotropic activities.
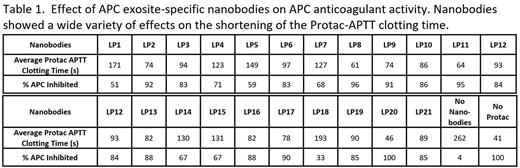
Results Of the total 1128 monoclonal phage clones screened in the library, 725 clones were identified as APC-PPACK binders. These clones were sequenced and 158 were found to be unique clones. Among them, 21 nanobodies belonging to 18 families were further characterized by their binding diversity. Overall, 3 clusters of nanobodies were identified based on their competition for APC exosites in BLI studies. APC functional assays for anticoagulant activity, histone H3 cleavage, and SEAP-PAR1 cleavage, were employed to understand the diversity of the nanobodies. These functional assays revealed 13 novel nanobody-induced APC activity profiles via the selective modulation of APC pleiotropic activities, with the potential of regulating specific mechanisms for therapeutic purposes. Within these, 3 nanobodies (LP2, LP8, LP17) inhibited all 3 APC functions. Five nanobodies (LP1, LP5, LP10, LP16, LP20) inhibited only 2 of the 3 functions. Mono-function inhibition specific to APC anticoagulation activity was observed only by 2 nanobodies (LP 9, LP11). LP 9 and LP11 are 2 of the nanobodies within the library that inhibited APC by >90% in a clotting assay (Table 1). The monospecific inhibition of APC anticoagulant activity could induce a pro-hemostatic effect in hemophilia and traumatic coagulopathy conditions. The molecular basis of APC's cytoprotective functions is based on its biased agonism of PAR1 by cleaving at R46. Interestingly, in R46/R41 cleavage ratio studies on SEAP-PAR1 cell lines, LP11 increased the APC pro-cytoprotective cleavage of R46 by up to 87%. The novel property of LP11 in enhancing R46 cleavage may potentially provide additional cytoprotection signaling and may potentially further benefit the outcome of these bleeding conditions.
Conclusions A library of APC exosite-specific llama nanobodies yielded 21 nanobodies that can selectively modulate APC anticoagulation and cytoprotection pathways. The non-competing nature of nanobodies from each cluster allows the possibility of combining nanobodies from different clusters to better understand and study the biology of APC. These nanobodies represent a tool kit of 13 categories of antibodies based on their cluster and activity profile and offer the opportunity to develop novel therapeutics for the treatment of an array of APC-associated indications including acute bleeding, hemophilia, ischemia, and sepsis.
Disclosures
Sim:Coagulant Therapeutics: Current Employment, Current equity holder in private company; BMS: Other: Spouse's employment. Shukla:Coagulant Therapeutics: Research Funding. Mallari:Coagulant Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Schneider:Coagulant Therapeutics: Consultancy; Gladiator Biosciences: Consultancy. Bauzon:Coagulant Therapeutics: Consultancy, Current equity holder in private company, Patents & Royalties: Patent; Catalent Biologics: Current Employment; Gladiator Biosciences: Consultancy, Current equity holder in private company. Hermiston:Jazz Pharmaceuticals: Consultancy; Gilead: Consultancy; Gladiator Biosciences: Current Employment, Current equity holder in private company, Patents & Royalties: Patents; SoBi: Consultancy; Kalivir Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Coagulant Therapeutics: Current Employment, Current equity holder in private company, Patents & Royalties: Patent. Mosnier:Coagulant Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal